CARTIBEADS TREATMENT OF ARTICULAR CARTILAGE LESIONS
Cartibeads treatment of articular cartilage lesions
“Cartibeads™ are bio-engineered cartilage grafts resembling native articular cartilage. These grafts, in the form of small cartilage beads, are produced from aggregated chondrocytes (cartilage cells) embedded in their self-secreted hyaline-like matrix, characterized by collagen II and glycosaminoglycan (GAG) presence. This is due to the patented Cartibeads™ method, which first guides chondrocytes through de-differentiation, to allow their expansion, and then re-differentiation, to regain their capacity to secrete a hyaline matrix. The final implantable product “Cartibeads™” consists of white-coloured beads with a diameter of ~1.5 mm.
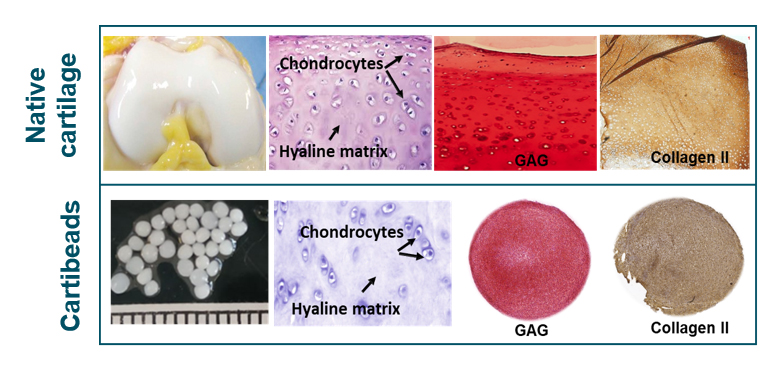
Cartibeads™ have self-integrative properties, which have been confirmed in several preclinical studies. Indeed, a perfect integration to the native cartilage and subchondral bone were observed upon implantation in minipigs. The Cartibeads™ repair tissue maintained hyaline-like quality and showed evidence of chondral zonal arrangement at 6 months follow-up. No signs of acute or chronic rejection were observed, proving the safety of the procedure. In all, these findings support Cartibeads™ potential for long-term cartilage repair.
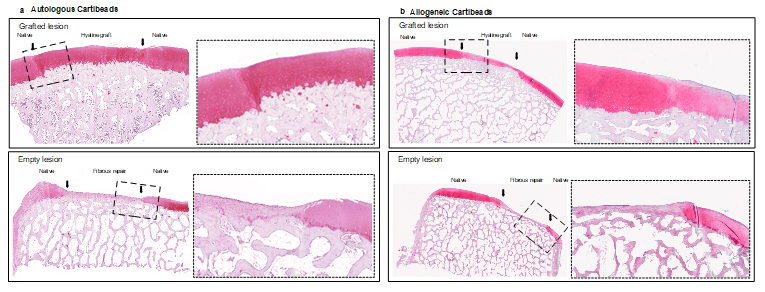
Cartibeads™ were first developed as an autologous product (made from the patient’s own cells). However, autologous treatment has several disadvantages, such as the need for 2 surgeries (biopsy harvest and mini-graft implantation) and long manufacturing (~8 weeks). Cartibeads™ were thus developed into an allogeneic product (made from donor cells). Allogeneic cartilage grafting, such as osteochondral allografts, has been routinely practiced for over 40 years with high success rate and no tissue rejection. The absence of rejection is explained by the lack of cartilage vascularization and the fact that cartilage cells are embedded in a thick hyaline matrix, and thus not exposed to the immune system. Altogether, Allogeneic Cartibeads™ offer a significant advancement in cartilage repair due to several advantages:
- Produced on-demand, quickly available
- Standardized and highly reproducible quality
- Do not require tissue compatibility
- Can treat large lesions and patients over age 55
- Only one surgery needed (no harvesting of patient cells)
More information regarding Cartibeads™ development and preclinical data can be found on our published articles :
- Kutaish, H., Bengtsson, L., Matthias Tscholl, P., Marteyn, A., Braunersreuther, V., Guérin, A., Béna, F., Gimelli, S., Longet, D., Ilmjärv, S., Dietrich, P. Y., Gerstel, E., Jaquet, V., Hannouche, D., Menetrey, J., Assal, M., Krause, K. H., Cosset, E., & Tieng, V. (2022). Hyaline Cartilage Microtissues Engineered from Adult Dedifferentiated Chondrocytes: Safety and Role of WNT Signalling. Stem Cells Transl Med, 11(12): p. 1219-1231.
- Kutaish, H., Tscholl, P. M., Cosset, E., Bengtsson, L., Braunersreuther, V., Mor, F. M., Laedermann, J., Furfaro, I., Stafylakis, D., Hannouche, D., Gerstel, E., Krause, K. H., Assal, M., Menetrey, J., & Tieng, V. (2023). Articular Cartilage Repair After Implantation of Hyaline Cartilage Beads Engineered from Adult Dedifferentiated Chondrocytes: Cartibeads Preclinical Efficacy Study in a Large Animal Model. Am J Sports Med, 51(1): p. 237-249.
- Kutaish H, Bengtsson L, Boudabbous S, Lazeyras F, Courvoisier S, Braunersreuther V, Hammer SE, Hannouche D, Ménétrey J, Tieng V, Tscholl PM. Allogenic Bioengineered Cartilage Achieves Hyaline Cartilage Repair in a Large Animal Model: A Promising Step Forward. Am J Sports Med. 2025 Jun;53(7):1641-1649. doi: 10.1177/03635465251331224. Epub 2025 Apr 27. PMID: 40289476; PMCID: PMC12125493.